40. The Chemistry of Food Coloring
Chemical Structure of Food Colouring
Food coloring molecules are organic compounds that have at least one chromophore and a conjugated system, which is a structure with alternating double and single bonds between atoms. Chromophores in food coloring dye are responsible for giving the dye its colour. However, the chromophore must be part of a conjugated system as shown in the Figure 1 below.
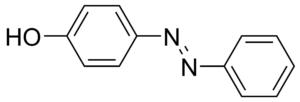
Figure 1 Molecular structure of an orange-yellow colored compound where the azo group ‘-N=N-’ acting as the chromophore is placed between two aromatic rings which have alternating single and double bonds
The chromophores absorb some wavelengths of light in the visible light spectrum, allowing complementary colours, which are opposite in the color wheel (Figure 2), to be transmitted. For example, if a chromophore were to absorb wavelengths of light corresponding to the color red, the light that will be transmitted, and that humans will be able to see, will be green. In our chemistry tuition, the concept of colors would be taught in greater details. The Crystal Field Theory (CFT) explains the mechanism behind chromophores absorbing and transmitting certain wavelengths of light.
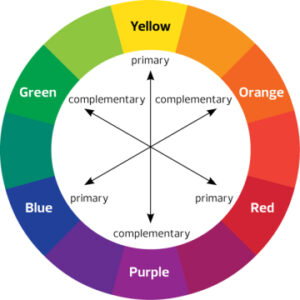
Figure 2 Color wheel
(https://www.peachpit.com/articles/article.aspx?p=2162084&seqNum=2)
Crystal Field Theory (CFT)
In an isolated atom, d-orbitals have the same energy. However, in a complex such as a chromophore, the d-orbitals do not have the same energy but are split into two sublevels with different energy levels. The difference in energy levels between the two sets of orbitals is defined as ∆”, the crystal field splitting energy. When the d-orbitals are partially filled, an electron can transition from the lower-energy set of d-orbitals to the higher-energy set of d- orbitals. For this transition to occur, a photon of light first needs to be absorbed. The ∆” affects the minimum amount of energy the photon of light absorbed needs to have for the electron to be able to transition to the higher energy level. The greater the size of ∆”, the more energy that is required. The size of ∆” is affected by many different factors, including the identity and oxidation state of the ion, and the geometry of the complex ion. Our chemistry tutor with his wealth of knowledge in the area of inorganic chemistry would break up these complex concepts into something simple for students to understand. When the size of ∆” changes, the energy of the photons of light absorbed changes and hence the wavelengths of light absorbed changes. Thus, the wavelength of light transmitted also changes and the colors that we can see changes.
Bleach, its Active Component and the Effect on Food Colouring
The bleach that is most commonly used for cleaning stains on clothes is a chlorine based bleach. In this bleach, the active ingredient used is sodium hypochlorite, NaClO, which is usually in a 3%-6% concentration in commercial bleaches. Sodium hypochlorite is a very strong oxidising agent. As taught in our chemistry tuition classes, when bleach interacts with the food coloring molecule, it accepts electrons from the molecule, oxidising the food coloring molecule. In azo dyes, this causes the azo linkage ‘-N=N-’ that acts as the chromophore to be cleaved. The different ∆” of the new compounds formed causes the compounds to absorb wavelengths of light that are outside the visible light spectrum. This causes all wavelengths of light within the visible light spectrum to be transmitted. When this happens, humans will see the light not as individual colors, but as white light. Hence, this causes the stain to appear colourless. This is just one of the many applications of chemistry in real life made interesting by our very own Chemistry tutor, Mr Jacky Wong.
Click HERE to read next
Chemistry Tuition Singapore @ MY CHEM CAFE
Principal Chemistry Tutor: Mr. Jacky Wong


